MBI Videos
Jay Newby
-
 Jay NewbyDespite being one of the fundamental cell structures, we know surprisingly little about the cytosol. Its physical properties are difficult to measure due to technical challenges: the means of spatially resolving viscosity, elasticity, flow, crowding, and confinement within cells that fluctuate and grow. Changes in macromolecular crowding can directly influence protein diffusion, reaction rates, and phase separation. I will discuss new particle tracking tools and how we use them to quantitatively measure the physical state of the cytosol by studying the three-dimensional stochastic motion of genetically expressed fluorescent nanoparticles (GEMs). Using these particle probes, we find that the physical properties of the cytosol vary significantly within and between cells, indicating that the fundamental state of the cytosol is a key source of heterogeneity within genetically identical cells.
Jay NewbyDespite being one of the fundamental cell structures, we know surprisingly little about the cytosol. Its physical properties are difficult to measure due to technical challenges: the means of spatially resolving viscosity, elasticity, flow, crowding, and confinement within cells that fluctuate and grow. Changes in macromolecular crowding can directly influence protein diffusion, reaction rates, and phase separation. I will discuss new particle tracking tools and how we use them to quantitatively measure the physical state of the cytosol by studying the three-dimensional stochastic motion of genetically expressed fluorescent nanoparticles (GEMs). Using these particle probes, we find that the physical properties of the cytosol vary significantly within and between cells, indicating that the fundamental state of the cytosol is a key source of heterogeneity within genetically identical cells. -
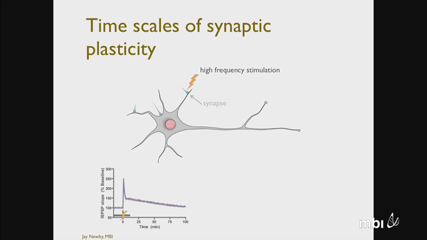 Jay Newby
Jay NewbyA key component in the cellular mechanisms underlying learning and memory involves the distribution and delivery of mRNA to synaptic sites in dendrites. A minimal three-state random intermittent search model of motor-driven mRNA transport is developed to explore the question of why motor-driven mRNA are observed moving bidirectionally. The model is analyzed by computing the probability an mRNA is delivered to a synaptic target and the average delivery time (MFPT). It is found that if the branched geometry of the dendrite is ignored, a purely unidirectional transport strategy will result in the smallest MFPT at any given delivery probability. The branched geometry of the dendrite is then incorporated into the model, and it is shown that a phase transition exists for a critical delivery probability where bidirectional strategies improve the corresponding MFPT. To further explore the impact of motor-driven transport behavior on mRNAdelivery, the three-state model is extended to include a detailed, biophysical model of a multimotor complex coordinated through a tug-of-war. The model is analyzed to explore how various measurable, physical quantities, such as adenosine triphosphate, can be tuned to optimize cargo delivery.
-
 Jay NewbyI will present theoretical support for a hypothesis about cell-cell contact, which plays a critical role in immune function. A fundamental question for all cell-cell interfaces is how receptors and ligands come into contact, despite being separated by large molecules, the extracellular fluid, and other structures in the glycocalyx. The cell membrane is a crowded domain filled with large glycoproteins that impair interactions between smaller pairs of molecules, such as the T cell receptor and its ligand, which is a key step in immunological information processing and decision-making. A first passage time problem allows us to gauge whether a reaction zone can be cleared of large molecules through passive diffusion on biologically relevant timescales. I combine numerical and asymptotic approaches to obtain a complete picture of the first passage time, which shows that passive diffusion alone would take far too long to account for experimentally observed cell-cell contact formation times. The result suggests that cell-cell contact formation may involve previously unknown active mechanical processes.
Jay NewbyI will present theoretical support for a hypothesis about cell-cell contact, which plays a critical role in immune function. A fundamental question for all cell-cell interfaces is how receptors and ligands come into contact, despite being separated by large molecules, the extracellular fluid, and other structures in the glycocalyx. The cell membrane is a crowded domain filled with large glycoproteins that impair interactions between smaller pairs of molecules, such as the T cell receptor and its ligand, which is a key step in immunological information processing and decision-making. A first passage time problem allows us to gauge whether a reaction zone can be cleared of large molecules through passive diffusion on biologically relevant timescales. I combine numerical and asymptotic approaches to obtain a complete picture of the first passage time, which shows that passive diffusion alone would take far too long to account for experimentally observed cell-cell contact formation times. The result suggests that cell-cell contact formation may involve previously unknown active mechanical processes.
